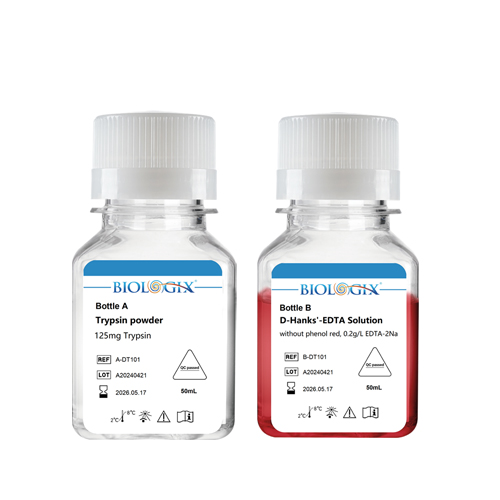
Biologix Trypsin is a type of serine hydrolase that cuts the carboxyl side of lysine and arginine residues in polypeptide chains, hydrolyzes intercellular proteins, and disrupts intercellular junctions, thereby disintegrating tissues or walled cells into individual cells. The enzyme solution is sterile and can be used directly in the digestion of cultured cells or the digestion of some tissues. When using trypsin, the concentration, temperature, and time should be controlled to avoid over-digestion and cell damage.
Usage method: Before use, under sterile conditions, transfer the solution from B bottle to A bottle, gently shake to mix well, and the trypsin will be obtained.
Storage conditions: The solution should be stored upon receipt at 2-8℃ .The product is stable for 24 months, repeated freezing and thawing are needed to be avoided.
Product performance:
1.Digestion time: The digestion time of Biologix's trypsin solution is similar to that of Brand G, but it is superior to that of Brand P in terms of time.
2.Proportion of cell density increase: After digestion with Biologix's trypsin solution, the growth multiple of different cell densities was similar to that of Brand G, especially showing a significant growth advantage on TM4 cells.